A Gallup poll last month indicated that 58 percent of Americans think marijuana should be legal. Currently, it is available for medicinal purposes in 20 states and the District of Columbia. Additionally, it is legal for recreational purposes in Colorado and Washington.
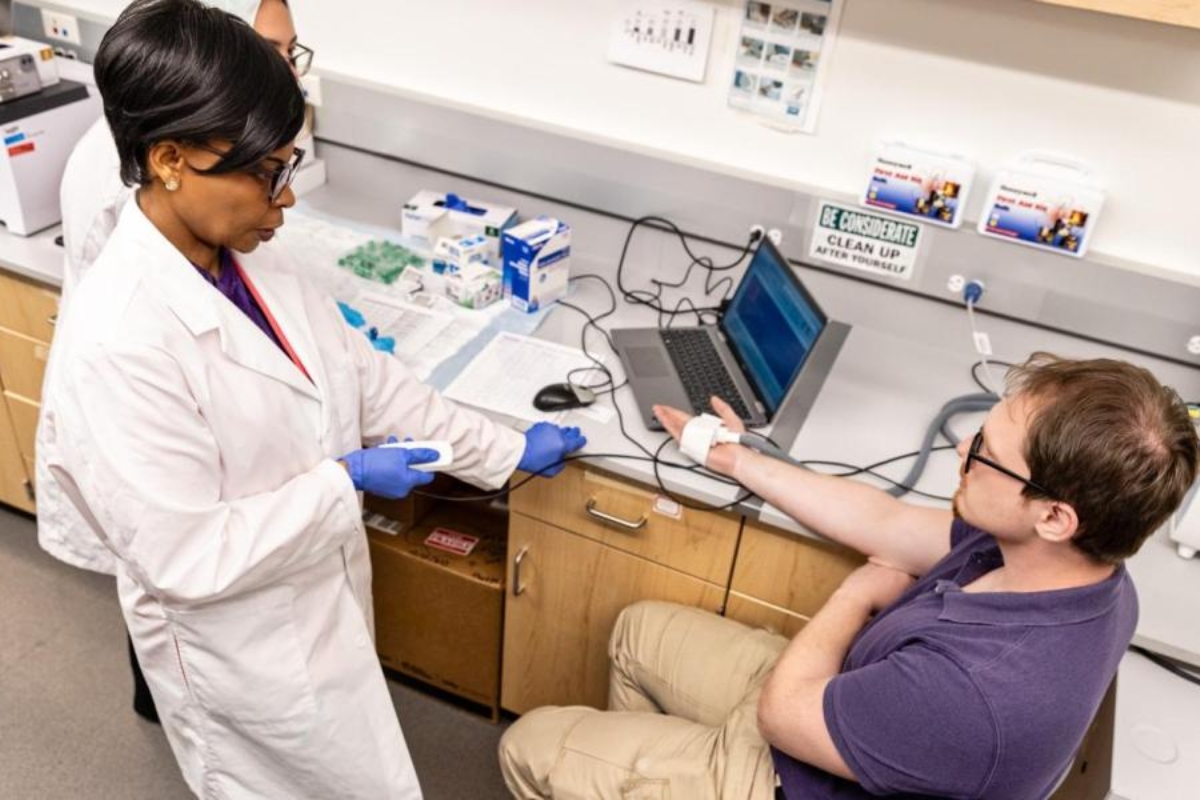
In 1970, Congress classified marijuana as an illegal Schedule I drug, a title usually reserved for street drugs like heroin. This classification has been an obstacle for scientists studying its medical effectiveness. A report published last year by the Mayo Clinic states that the classification is only political and has resulted from ignoring 40 years of research. In the report, Dr. J. Michael Bostwick vouches for research showing that throughout our body we have cell receptors for marijuana’s active ingredients.
Cannabinoids are natural substances that bind to these receptors, setting off pathways for a wide range of body processes. These receptors are said to impact the autonomic nervous system, immune system, gastrointestinal tract, reproductive system, cardiovascular system and endocrine network, collectively dubbed the endocannabinoid system. Epilepsy, alcoholism, and post-traumatic stress disorders have been associated with disruptions in these system, and findings suggest patients may benefit from marijuana’s ingredients.
Diane E. Hoffmann and Ellen Weber, legal experts at the University of Maryland, wrote in The New England Journal of Medicine, “Medical experts emphasize the need to reclassify marijuana as a Schedule II drug to facilitate rigorous scientific evaluation of the potential therapeutic benefits.”
Many experts are currently working on different strains of marijuana that remove THC, the component with psychoactive properties. Sativex is a drug currently in Phase 3 trials in the United States. The drug is an amalgam of CBD and THC in a ratio that minimizes the appetite stimulation, drowsiness and anxiety induced by THC while magnifying painkilling and anti-tumor properties. However, some patients such as cancer patients benefit from the appetite inducing properties of THC. It is clear there is still a lot of research to be done.
“We believe that physicians should clearly explain to their patients that medical marijuana is not approved by the Food and Drug Administration and that it is not a standardized or purified product,” Dr. Herbert D. Kleber of Columbia University and Dr. Robert L. Dupont of Georgetown Medical School wrote last year in The American Journal of Psychiatry.
Without easy access to researchers, such medicinal studies are difficult, despite the potential of medicinal marijuana. Patients and doctors need to work together to ensure it is used appropriately.
by Fatima Ali